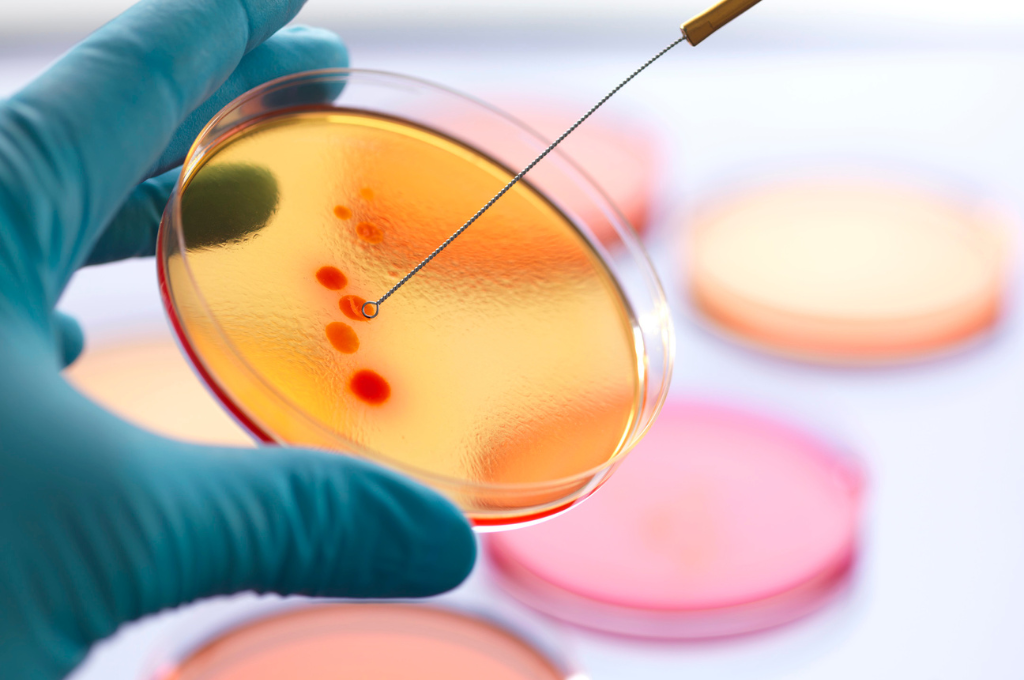
The spread of antibiotic-resistant bacteria is a growing problem in modern medicine. Bacteria that have developed resistance mechanisms against traditional antibiotics are becoming increasingly dangerous and difficult to combat. It is therefore necessary to search for modern compounds that are effective against these dangerous pathogens.
Antibiotic-resistant microorganisms, also known as “superbugs”, develop their resistance through genetic mutations or gene transfer between different bacterial species. As a result, traditional antibiotics, which have been an excellent weapon against bacterial infections for decades, are losing their effectiveness against new resistant strains. These types of bacteria can cause dangerous infections that are often hard to keep under control and can even lead to the death of the patient, as they are able to survive the use of even the most effective antibiotics, making the treatment of such infections problematic. These strains are often found in hospitals, where patients are at a higher risk of contracting an infection.
Risk factors for the development of superbugs
One of the main factors behind the problem is our inappropriate approach to antibiotics. Frequent and uncontrolled use of antibiotics leads to the development of bacterial resistance to such drugs. Patients who stop taking antibiotics before completing their treatment or using them without consulting their doctor also contribute to the selection of resistant bacteria. Hospital-contracted infections play an important role in the spread of antibiotic-resistant microorganisms. In hospitals, where patients have a weakened immune system, antibiotic-resistant bacteria can easily spread from patient to patient, creating the above “superbugs”.
Another factor is the use of antibiotics in raising animals for meat. Antibiotics are commonly used to speed up growth and prevent disease in farm animals. Zoonotic bacteria can develop resistance to antibiotics and can then be transferred to humans through food consumption or contact with the animal.
A limited number of new discoveries in the field of pharmacology is another factor. Despite the rise in antibiotic-resistant bacteria, innovative antibiotics are rarely developed. Pharmaceutical companies point to the low profits associated with producing antibiotics in comparison with other medicines.
The need to develop advanced formulations
It is therefore necessary to develop new and innovative antibiotic formulations that are effective against antibiotic-resistant bacteria. Over the past few years, scientists around the world have been conducting intensive research into new compounds that could be useful in the treatment of infections caused by these dangerous pathogens.
One novel compound with therapeutic potential is cresomycin, a macrobicyclic compound that can interact with the bacterial ribosome. The compound has been developed by a team of researchers including Andrew Myers, Kelvin Wu and Ben Calhoun Tresco from the American Harvard College. Cresomycin has also been the subject of international patent application WO2023205206A1 entitled “Lincosamides and their uses”, benefiting from the priority of the so-called provisional US patent application US202263332993P.
Cresomycin has proved effective against both Gram-positive and Gram-negative bacteria, including multi-drug resistant bacterial strains such as Staphylococcusaureus, Escherichia coli and Pseudomonasaeruginosa.

Source: Cresomycin; Gillmore Health News, “Antibiotic Breakthrough: Cresomycin Overcomes Multi-Drug Resistant Bacteria by Targeting Ribosomal Methylation”.
With antibiotic resistance on the rise, cresomycin seems to be a promising compound to combat bacterial infections. In addition, it appears to have a low level of toxicity to the human body, which could make it a potentially safe compound for clinical use.
Protecting the invention
The question thus arises as to whether, in the light of the pending international application, cresomycin will be the active substance used for the treatment of infections caused by antibiotic-resistant bacteria and consequently will be widely available on the pharmaceutical market. It is worth noting that in December 2023 the Applicant indicated the European territory as the desired territory for patent protection. One must mention that the US Patent Office, as an international search authority, carried out an international patent search in which it identified 4 documents whose contents do not appear to constitute an obstacle to the granting of a patent with respect to the grounds of patentability. In particular, they do not contain identical or similar information to the international patent application, nor do they disclose any information that would indicate the obviousness of the invention that is the subject of the said application.
The Applicant’s declaration to file the international application with the European Patent Office as part of the so-called Euro-PCT procedure within 31 months from the date of the first correct application (priority date) in order to obtain the broadest market monopoly appears to be a mere formality in view of the above-mentioned application.
Obtaining patents for new chemical structures with potential antibacterial activity in the context of antibiotic resistance is particularly important from the point of view of both patients and pharmaceutical companies. Granting the marketing authorisation will certainly lead to a wider availability of such drugs on the global pharmaceutical market.
Collaboration across sectors is essential
The fight against antibiotic-resistant bacteria requires an integrated approach that combines the efforts of scientists, doctors, veterinarians and policymakers. It is necessary to reduce the overuse of antibiotics in human medicine and animal breeding, promote hygiene and introduce preventive measures in order to reduce the possibility of spreading drug resistant bacteria.
It is also important to educate the public about the responsible use of antibiotics and to encourage the search for alternative treatments for infections. Only through joint effort can we effectively stop the spread of antibiotic-resistant bacteria and ensure a secure future in our fight against infections.
Whether the over-concentration of patents in the hands of a small group of large pharmaceutical companies, colloquially known as “Big Pharma”, is limiting patient access to new therapies and stifling innovation in the field of antibiotics has been debated for years. It is therefore crucial to balance the interests of patients and pharmaceutical companies through appropriate regulation and incentives for public-private collaboration to promote the development of new antibiotics and broad access to the latest medical discoveries.